Resistance of Plasmodium falciparum to chloroquine, a cheap and well tolerated treatment and prophylaxis, emerged simultaneously in Southeast Asia and Central America during the early 1960s. Although chloroquine resistance took hold in Africa later, by the early 1990s it appears to have been widespread in East Africa; in 2000 Kenya banned its sale except by prescription. Resistance has also developed, sooner or later, to nearly all promising, long lasting drugs introduced as replacements to chloroquine for individual malaria prevention. In a clinical study done in 1998 with USAMRU support, Dr Bernhards Ogutu, of the Centre for Clinical Research, found that 35% of a study group of children in Nairobi had some degree of resistance to Fansidar (pyrimethamine and sulfadoxine).
|
-
Correlate in vitro and in vivo malaria drug resistance.
-
Map malaria drug resistance patterns across Africa.
-
Investigate molecular mutations in the DHFR and DHPS genes of P. falciparum that are predictive of clinical failure.
-
Develop laboratory capabilities and provide reference testing for East Africa.
Recent Work
In June, 2001 we began operation of the first in vitro drug testing facility in East Africa, at KEMRI in Nairobi. P. falciparum are cultured for two weeks and then exposed, in treated microtiter plates, to a battery of 16 pharmaceuticals. Resistant isolates are those that incorporate tritiated hypoxanthine, as compared to standard lines maintained at WRAIR. The system is automated and data are stored and analyzed using a custom designed program. The first results - from more than 400 specimens collected during the last two years - will be presented late in 2001. In 2002 we will begin systematic testing of specimens from throughout Kenya and from various sites in Uganda and Tanzania. Future elaborations will include testing drug combinations and correlating the in vitro results with clinical and genetic determinations of resistance. Patterns of resistance will be posted and updated on this web site.
| Folates: chloroquine, mefloquine, quinine, artemisinin, halofantrine, doxycycline, amodiquine, primaquine, atovaquine, tafenoquine. |
| Antifolates: dapsone, proguanil, sulfadoxine, pyrimethamine, chlorcycloguanil, cycloguanil |
We have begun a survey of point mutations in DHFR (51, 59, 108, 164) and DHPS (437, 463, 581, 631) in both freshly collected and archived specimens, which will be correlated with the in vitro results and, when available, clinical outcomes. Of 53 specimens collected from a remote area of southern Kenya, a single DHPS mutation (437) was found in 92%, but a triple DHFR mutation (51, 59, 108) was found in 87%, indicating a high level of anti-folate resistance. Both resistance markers appear to be widely diffused in Kenya.
AccomplishmentsThe malaria drug resistance project is proud to have established the first in vitro malaria drug sensitivity laboratory in Equatorial Africa. The project was the first to establish molecular malaria drug resistance data from Southern Kenya (Entasopia-Magadi).
Resistance to Resistance to anti-malarial drugs is emerging faster than new drugs can be developed. The Walter Reed Army Institute of Research is the world's leading public institution dedicated to anti-malarial drug discovery: it pioneered efficacious treatment of chloroquine resistant cases, developed mefloquine and halofanthrine, and was instrumental in the evaluation of most preventive measures now in use, including doxycycline and Malarone. The MRU plays a crucial role in the realistic evaluation of new drugs and drug combinations for safety and efficacy.Because western Kenya is subject to high malaria transmission rates - often higher than 100 inoculations per person per year - new prophylactic drugs can be evaluated in relatively small numbers of people.p; The highland areas, on the other hand, offer the opportunity to test therapeutic drugs in infected migrants under conditions that preclude reinfection
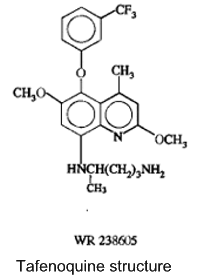
USAMRU most recently has evaluated tafenoquine, an 8-aminoquinoline analog of primaquine, that in preliminary testing has shown great promise as a safe, efficacious preventitive. Tafenoquine, which was developed at WRAIR and is being tested in partnership with Glaxo SmithKline, acts against the liver stages of the parasite, is better tolerated than primaquine, and is long-acting. Phase III trials are now underway in western Kenya as well as in Asia and Latin America.
Perform “proof-of-concept” (Phase II) studies and definitive (Phase III) trials of new drugs for malaria prevention and treatment.
Perform “proof-of-concept” (Phase II) studies and definitive (Phase III) trials with combinations of existing drugs for malaria prevention and treatment.
Provide on-going in vivo monitoring of drug-susceptibility to standard antimalarial drugs in Kenya.
Perform Phase II studies with a new intravenous artemisinin study (2003-4).
 Nearly all anti-malarials can be divided into two chemical classes, depending on
where they interfere in the parasite pathway to DNA synthesis: folates (for example,
chloroquine, primaquine), which inhibit the action
of dihydrofolate reductase (DHFR), and anti-folates (e.g., pyrimethamine,
sulfadoxine),
which interfere with dihydropteroate synthetase (DHPS). Mutations in the
genes coding for these two enzymes may develop under drug pressure and render
the parasite resistant. The primary objective of our
work is to map the extent of resistance in East Africa for a wide range of both
classes of drugs and to provide this information to public health
workers for the better prevention and treatment of malaria. To do this it
is necessary to establish systematic surveillance based on a consistent method
of in vitro testing.
Nearly all anti-malarials can be divided into two chemical classes, depending on
where they interfere in the parasite pathway to DNA synthesis: folates (for example,
chloroquine, primaquine), which inhibit the action
of dihydrofolate reductase (DHFR), and anti-folates (e.g., pyrimethamine,
sulfadoxine),
which interfere with dihydropteroate synthetase (DHPS). Mutations in the
genes coding for these two enzymes may develop under drug pressure and render
the parasite resistant. The primary objective of our
work is to map the extent of resistance in East Africa for a wide range of both
classes of drugs and to provide this information to public health
workers for the better prevention and treatment of malaria. To do this it
is necessary to establish systematic surveillance based on a consistent method
of in vitro testing.